Our Story
The beginning
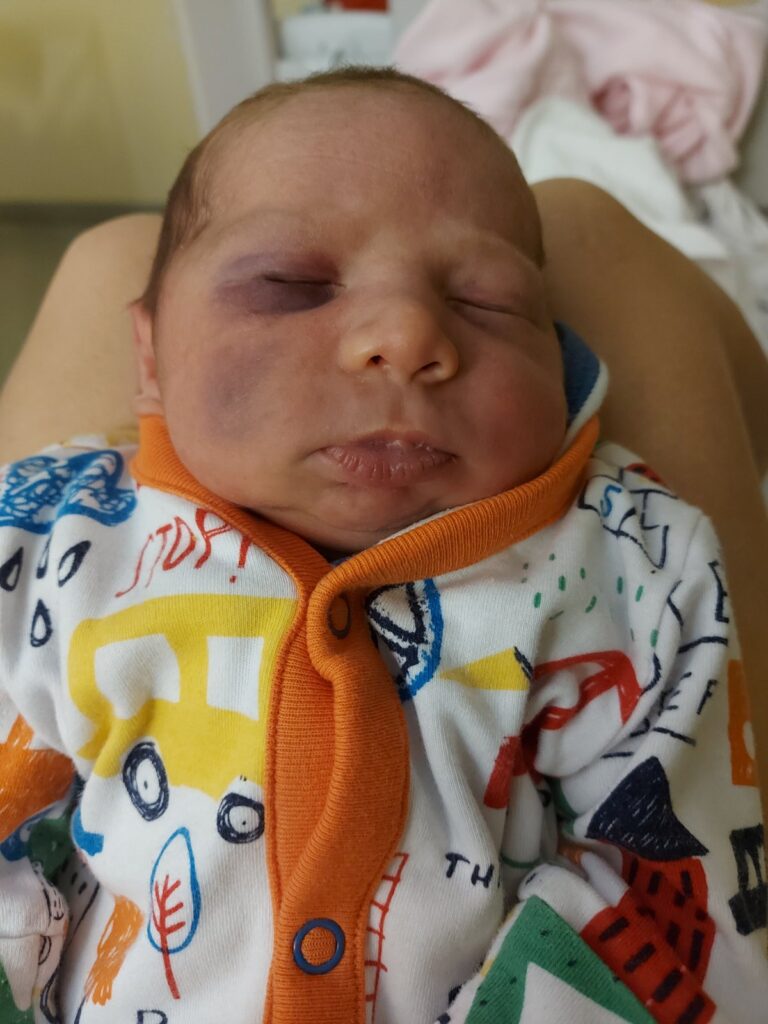
In September 2023, at 36 weeks, our long-awaited second son, Bertold, was born. He arrived a bit earlier than his due date, and for weeks his tiny face was marked by a hematoma (a “black eye”) he got during the difficult moments of labor. At the time, we didn’t know what role this injury would play in revealing a shocking diagnosis later on. We simply called him our little fighter—a name that still fits him today. After a few days in the hospital, we were able to take our child home and begin life as a family of four. Our happiness, however, didn’t last long. Bertold’s—and our family’s—struggle began very early. Because of the birth-related eye injury, we took him for an ophthalmology exam when he was one week old. Although they saw no damage that gave cause for concern, they recommended we schedule a cranial ultrasound as a precaution. That’s how, on November 13, 2023—when Bertold was one and a half months old—we ended up having a cranial ultrasound. On the ultrasound, the radiologist noticed an abnormality (a discrete increase in inhomogeneous reflectivity). The following week, our little boy was examined in the Developmental Neurology Department at Szent Margit Hospital. The ultrasound performed there also confirmed that there was some kind of lesion on the left side, in the area of the medial thalamic nuclei.
After returning home from the two-week hospital stay, we continued at home the exercises we’d learned in Developmental Neurology to strengthen Bertold’s attention, and we anxiously awaited the scheduled MRI in January.
Diagnosis
At the time of the first MRI (2024-01-03), Bertold was 3 months old. That was when what we had thought was only a nightmare became reality. It was confirmed that the abnormality seen on the ultrasound posed a real danger. The diagnosis presumed an optic glioma (a tumor of the optic nerve) in Bertold. He was placed under close neurological and ophthalmological follow-up, and a repeat MRI was scheduled for March. At the same time, Bertold’s care was taken over by the Oncology Department of the Children’s Clinic on Tűzoltó Street.
The wait felt very long in this uncertainty, but we kept hoping we’d get good news in March.
However, the March diagnosis was even more devastating. The tumor had shown significant growth — it had increased more than fivefold in just two months. The doctors told us that it was almost certain Bertold would lose his vision. By that time, he was already experiencing serious vision problems and struggled with intense eye movement (both rotational and horizontal nystagmus), accompanied by attention difficulties. Our concern about the possible onset of hydrocephalus also grew to an overwhelming level.
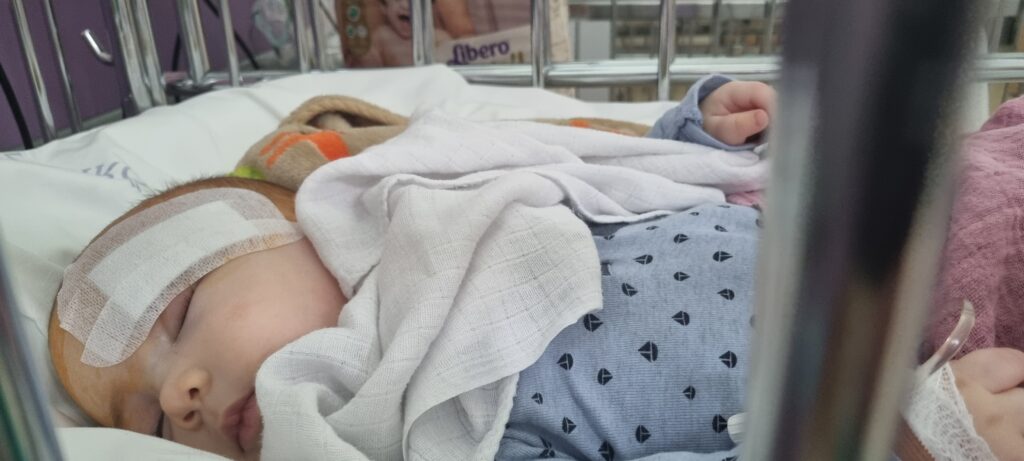
Immediately after the MRI, a neurosurgical consultation was scheduled at the National Institute of Mental Health, Neurology, and Neurosurgery. On March 21, 2024, a biopsy and lesion debulking were performed. According to the medical opinion, the tumor could not be surgically removed due to its location. After being discharged from the hospital, the start of further treatment was made dependent on the histopathological results. Once we received the pathology report, it was revealed that Bertold’s tumor was a pilocytic astrocytoma with a BRAF V600E mutation.
The first targeted treatment
Following the biopsy results, we began targeted drug therapy in April. By then, as parents, we had come to “accept” the idea that our little boy would never experience the joy of sight. We had one goal left—to ensure he would reach adulthood and, though blind, grow up to be a healthy adult.
At that time, our exclusively breastfed baby received his first medications in April 2024.
Alongside oncology and neurosurgical care, neurological and genetic evaluations were carried out. Molecular genetic testing from blood and a buccal swab did not confirm an NF1 (neurofibromatosis) background for the glioma, a genetic condition that would increase the likelihood of an optic nerve tumor.
Along with the medical examinations, we tried to emphasize Bertold’s continuous development (vision and cognitive functions). He primarily received vision therapy, since at that time his motor development showed only minimal delay. During this period, in addition to a rapidly increasing head circumference, Bertold had pronounced strabismus. Patching his eyes was part of our daily routine.
With the drug therapy and continuous developmental work, we managed to create a livable, relatively “normal” routine. What’s more, the MRI performed in the third month after starting therapy reported a slight decrease.
New, devastating news arrived on the first anniversary of the start of our ordeal. The next 3-month MRI showed further growth. Our doctors still recommended continuing the drug therapy (at that time, it was considered the most promising available treatment for this disease), and we anxiously awaited the following MRI—whose report, however, described further progression.
By then, the tumor’s growth had caused new symptoms. Bertold developed epileptic seizures, which made antiepileptic medication necessary from January 2025. Based on EEG examinations, the diagnosed focal epileptic dysfunction could only be controlled with the simultaneous use of several antiepileptic drugs.
Chemotherapy
Endocrine evaluations were also started, since the tumor and its location can affect endocrine functions.
On the next MRI, the radiologists again observed growth. The repeated progression justified a change in therapy. Thus, on May 14, 2025, Bertold began chemotherapy. After port placement (through which chemotherapy is administered to pediatric patients), the first infusion was given. Bertold handled the first treatment very poorly. He trembled, cried constantly, and was in continuous pain. He was therefore sent for another neurosurgical check to rule out increased intracranial pressure as the cause of the change in behavior. Fortunately, the CT scan did not confirm elevated intracranial pressure. The etiology of the tremor in his upper limbs remained uncertain. After a longer hospital stay, Bertold improved. Chemotherapy continued, supported by adjunct medications.
Tovorafenib
Because Bertold is still very young, neither radiotherapy nor proton therapy is a recommended option. Like our doctors, we know we need to buy time and seize every opportunity to slow the tumor’s growth. Even before starting chemotherapy, our doctor prepared us for the fact that this treatment rarely proves effective for this type of tumor, but given the circumstances, we had no choice. We hoped to be among the rare exceptions while also searching for additional options. That’s how we found Tovorafenib. This experimental, orally administered, selective pan-RAF kinase inhibitor is a targeted anticancer drug that is particularly effective against tumors with mutations in the BRAF gene—like Bertold’s.
Because Bertold is still so little, his condition may evolve very differently depending on whether we can stop the tumor from spreading. At present, both his motor development and cognitive abilities lag behind his peers, but his development—though slower—continues steadily. His near vision exceeds our expectations, but unfortunately his visual acuity and functioning at distance are very limited (until he starts speaking, a definitive ophthalmological diagnosis cannot be made). Despite his young age, Bertold has faced a great deal. He has undergone brain surgery (biopsy), countless blood draws and infusions, blood transfusions, seven MRIs under anesthesia, CT examinations, and seemingly endless hospital stays. Although he is not yet walking, he is a true bundle of energy—a real hero, like his fellow fighters. But even the greatest heroes sometimes need help. Please be the one who helps him—and us—get through these 12 months, after which targeted drug therapy will also become available at home for Bertold, which is currently our greatest hope.
