Oncology—and pediatric oncology in particular—is undergoing a major paradigm shift. It is becoming a field of precision medicine, where treatments are tailored to the individual patient. In cancer care, terms like first-line, second-line, etc., are often used; essentially, they indicate the sequence of attempts to regain control over the tumor.
First-line therapy:
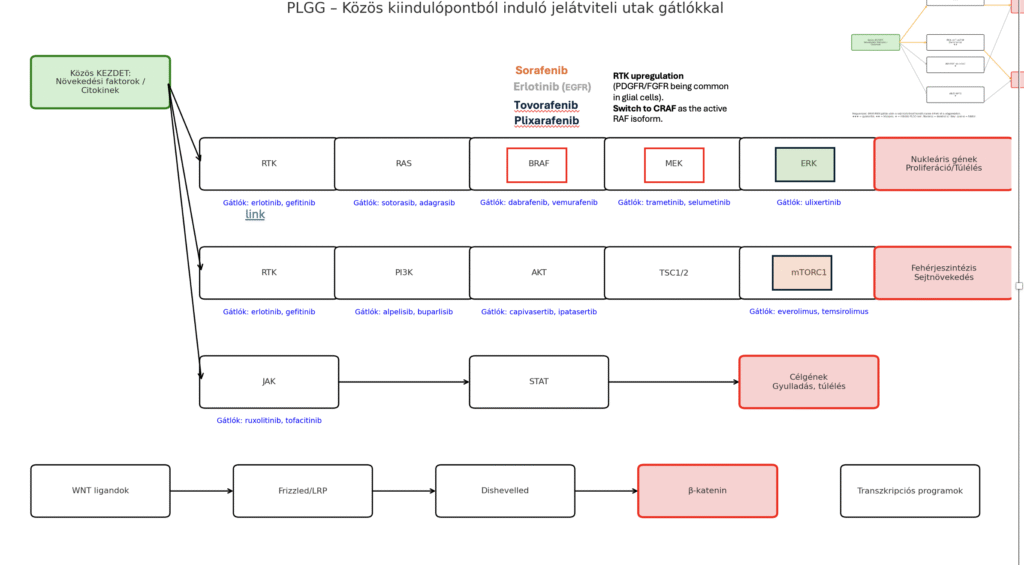
After the biopsy, the presence of the BRAF V600E mutation was confirmed. The diagram below shows the possible routes by which the “divide” signal can reach tumor cells. The most common pathway is the RAF/MEK pathway, which is consistent with the BRAF V600E finding. Therefore, the first-line treatment consisted of blocker medications that act along this branch—the red markings on the diagram indicate where the intervention takes place. The drug pair was approved in 2022. One had to be taken every 24 hours and the other every 12 hours (for us, 7 a.m. and 7 p.m.). Compared with chemotherapy, we faced far fewer and milder side effects—primarily various eczematous patches. At first the blood count was checked weekly, later monthly.
.
Second line
Roughly 8 months passed when the first MRI again showed spread, and unfortunately the MRI two months later indicated treatment failure—the therapy was no longer able to control the tumor. After extensive research and consultation, we tried replacing the RAF inhibitor with another RAF inhibitor. By about mid-April we reached the target dose. Like the original RAF inhibitor, this medicine was taken every 12 hours; however, unlike the previous drug, it did not require fasting (no food 2 hours before and 1 hour after), which matters a lot for a 2-year-old child. Unfortunately, the trade-off was greater liver strain: our values stayed within limits but hovered in the upper range. In the vast majority of cases, the division signal still travels along the upper pathway; resistance to the drugs likely developed. Therefore, this pathway remained the primary target. In both therapies we used first-generation inhibitors; fortunately, second-generation inhibitors have since been developed that can curb tumor growth much more effectively.
Third line
On May 12 we received the call that this therapy had failed as well—new spread was visible on the MRI—and immediate chemotherapy was recommended. The next day, during a minor procedure, Bertold’s port was implanted. It’s a small titanium box under the chest skin, with its end leading into a central vein; chemotherapy is administered through it, which both reduces pain and allows more effective delivery. Until recently doctors typically chose either chemotherapy or targeted therapy, but newer trials combine the two; in Bertold’s case, one inhibitor drug was continued. Although chemotherapy loses control in about 80% of cases, we hoped this “twist” would improve the odds.
Chemotherapy protocol
Chemotherapy for glioma typically has an induction phase and a maintenance phase. During induction we received treatment weekly, usually on Mondays, and we quickly learned the routine of keeping a little whirlwind still for 3–4 hours. We received a combination of Carboplatin and Vincristine, likely one of the strongest combinations. With these agents, white blood cells—the infection fighters—are “collateral damage,” but counts usually don’t fall as low as during leukemia treatment, so a subcutaneous growth-factor injection can be given to boost low counts; we used it as needed every 2–3 weeks based on labs. After the initial difficulties, a rhythm set in: Monday—chemo, Wednesday—nadir (fatigue, fussiness), Friday—recovery. The toughest part was that any fever meant immediately going to the ward, because if the white count was low, a serious condition could develop fast; we also had to avoid group settings. By the end of July, the MRI partly met our hopes: there was a minimal decrease in the previously spreading area, but a new enhancing focus appeared elsewhere—an early sign of further spread.
Fourth line
I’d like to devote a separate section to the story of tovorafenib; for now, suffice it to say that—after great difficulty—we managed to import the first boxed medicine, given orally once a week. So far we’ve seen fatigue in the days after dosing, and the first skin symptoms (eczema) seem to be appearing. It’s important to schedule periodic breaks so the little body can grow, because the medicine effectively inhibits intercellular signaling. We’re continuing chemotherapy in a maintenance phase (one treatment per month). The already sparse hair was lost in the first 10 weeks, but it looks like we’ll get it back in this phase. 🙂
As I wrote at the beginning, this whole field is on the brink of explosive progress. Almost every country is trying to find the ideal approach for these disease types.
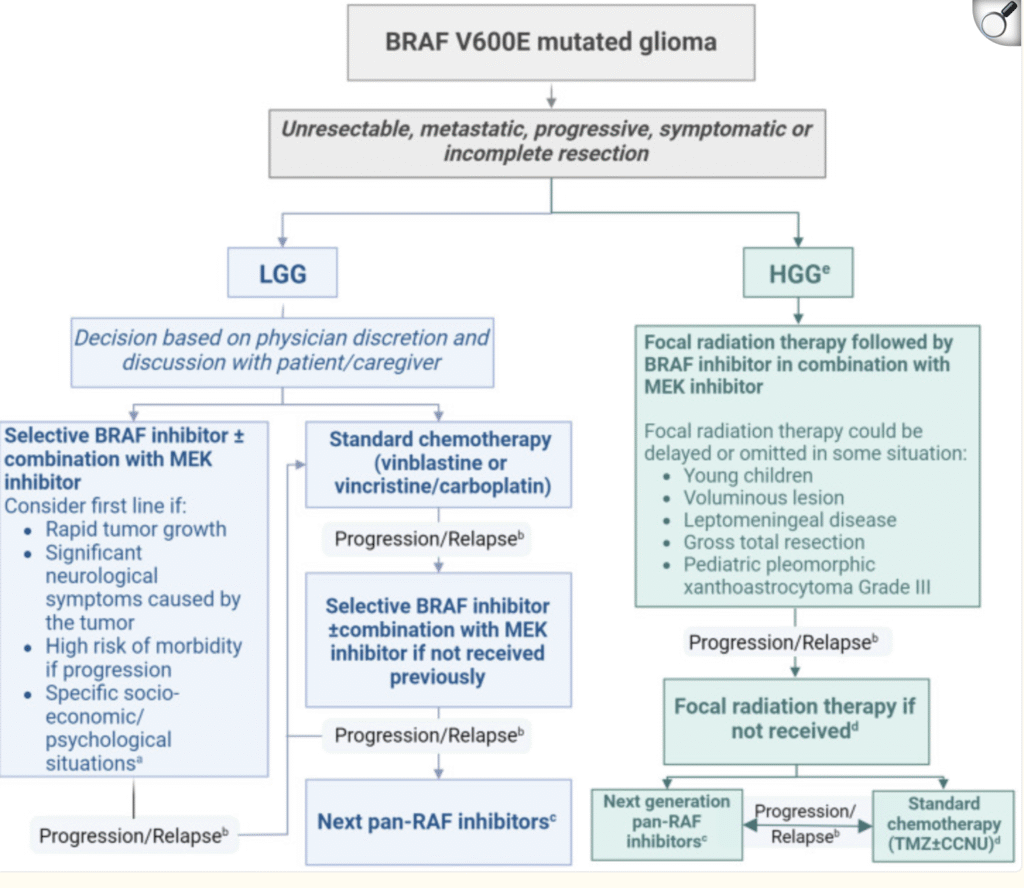
The above aligns very well with the Canadian consensus. Perhaps that protocol summarizes most precisely what we are experiencing.